
Is prepared to grant authorized persons access to their medical records.1 positive rapid tests (Abbott-DetermineTM HIV-1/2 or Sensa (Seyama Solutions, SA)) and an additional confirmatory ELISA (Enzygnost anti-HIV-1/2 Plus).2 positive rapid tests (Abbott-DetermineTM HIV-1/2 and Sensa (Seyama Solutions, SA)) or.Has a compatible clinical picture of TB according to South African guidelines with the intention to treat.Inclusion Criteria (HIV positive patients): Is prepared to grant authorized persons access to their medical record.
#Arm negative tb test trial#
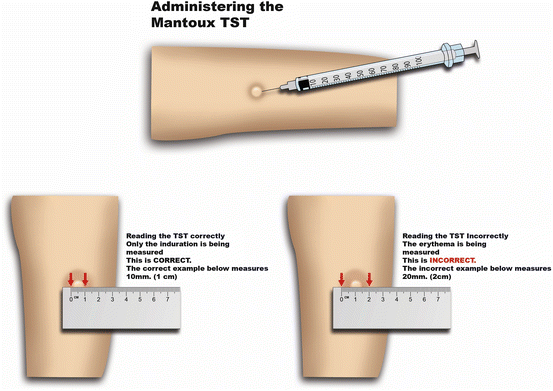
Tuberculin PPD RT 23 SSI, to compare the induration response of C-Tb with the in-vitro IFN-γ response measured at screening using the QuantiFERON®-TB Gold In Tube assay, to correlate the induration response to the initial CD4+ counts in HIV positive patients and to record all adverse events (local and systemic) occurring within 28 days after application of the agents The secondary objectives of the trial is to compare the induration response of C-Tb with the induration response of 2 T.U.

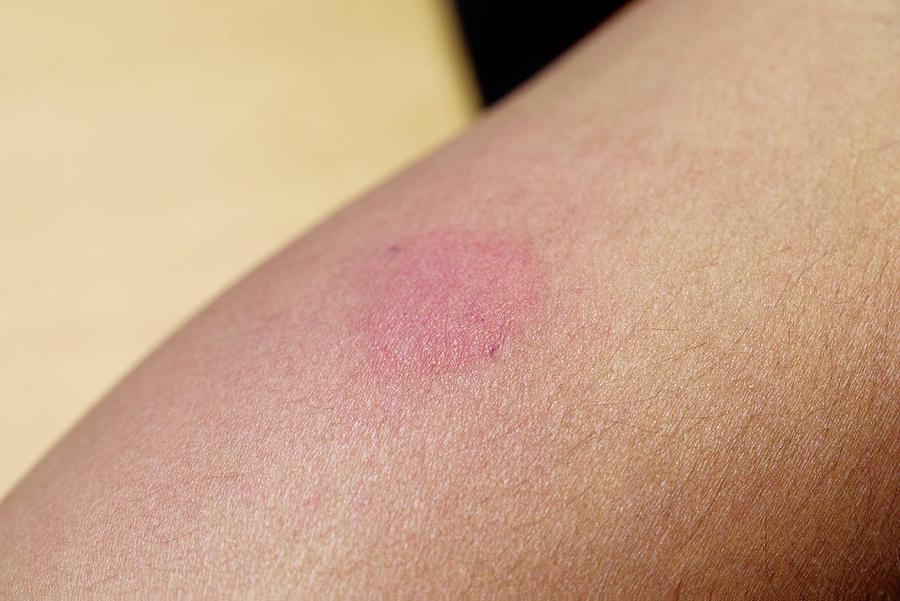
tuberculosis) who have an induration response < cut-off after a C-Tb test.Īn optimal cut-off point of being infected will be determined by combing the results from the present sensitivity study with those from a parallel specificity study in (BCG vaccinated) individuals with no previous exposure to M. Similarly the specificity of the C-Tb test is defined as the relative frequency of subjects in a healthy population (i.e., no exposure to M. The primary objectives are to assess the sensitivity of the C-Tb test as a function of the cut-off value (i.e., the smallest size of induration measured in mm resulting in a positive outcome of the C-Tb test) when the test is administered intradermally by the Mantoux technique to HIV negative adult patients recently diagnosed with active TB and to assess the sensitivity of the C-Tb test as a function of the cut-off value (i.e., the smallest size of induration measured in mm resulting in a positive outcome of the C-Tb test) when the test is administered intradermally by the Mantoux technique to HIV positive adult patients recently diagnosed with active TB The sensitivity is defined as the relative frequency of patients with an induration response ≥ cut-off in TB patients. The C-Tb and 2 TU Tuberculin PPD RT 23 SSI agents are given concomitantly to each volunteer in the RIGHT AND LEFT forearms according to a double blind randomisation scheme. Two groups of adult patients recently diagnosed with active TB will be investigated patients in the main group will NOT have a co-infection with HIV and patients in the second group will have a co-infection with HIV. Tuberculin PPD RT 23 SSI in the other arm). (Each volunteer receives the C-Tb agent in one arm and 2 T.U. The trial is designed to investigate the sensitivity of C-Tb using various sizes of cut-off of induration in a double blind randomised, split-body study comparing 0.1 µg/0.1 mL C-Tb with the reference agent 2 T.U. Why Should I Register and Submit Results?.


 0 kommentar(er)
0 kommentar(er)
